Featured
- Get link
- X
- Other Apps
Transition Metals Conduct Electricity
Transition Metals Conduct Electricity. All answers (4) the conductivity of metal in solution depends on how much the metal is soluble in solution. Discover things that you didn't know about transition metals conduct electricity on echemi.com.
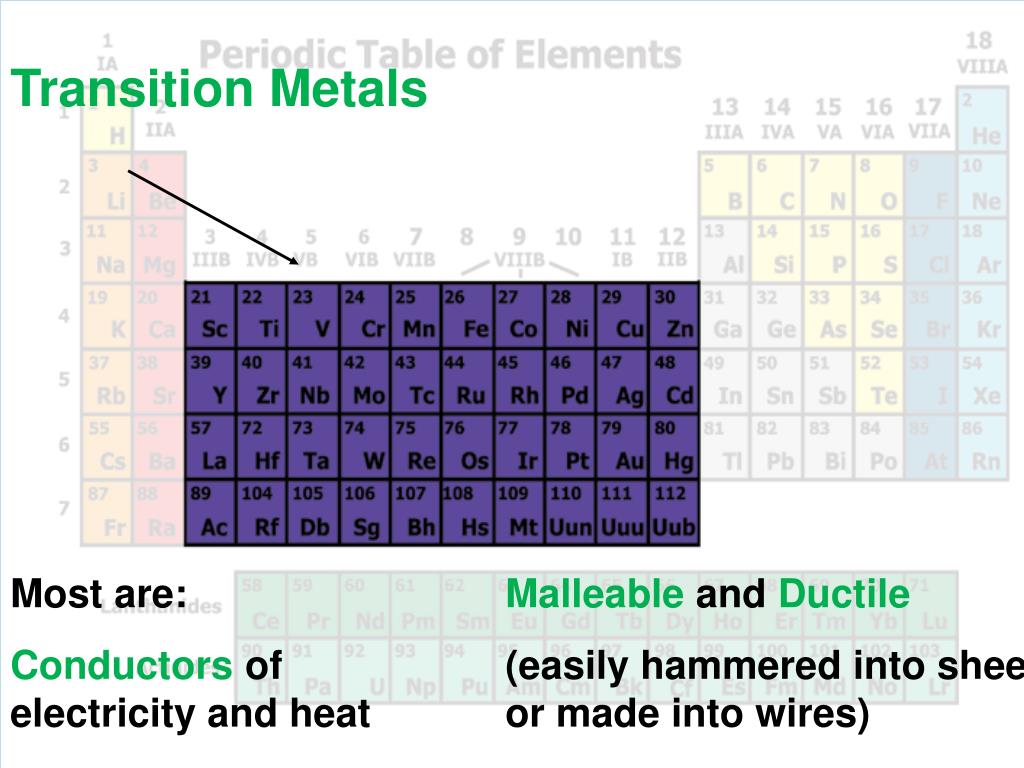
Transition metals have similar properties, and some of these properties are different from those of the metals in group 1. They can conduct electricity and are good conductors of. In chemistry, the term transition metal (or transition element) has three possible definitions:
These Electrons Are Why The Metal Can Produce Electricity Because Electron Migration Is How Current Electron Flow Is Generated.
This moving sea of electrons enables the metal to conduct electricity and move freely among the ions. They have relatively high densities. The most highly conductive metals are silver, copper.
1— In A Recent Paper Certain Property Of The Transition Metals Ni, Pd, And Pt And Of Their Alloys With Cu, Ag, And Au Have Been Discussed From The Point Of View Of The Electron Theory Of Metals Based On Quantum Mechanics.
While the term transition has no particular chemical significance, it is a convenient name by which to distinguish the similarity of the atomic structures and resulting properties of the elements so. Silver, copper, and gold are the best conductors of electric current in metals. Transition metals have similar properties, and some of these properties are different from those of the metals in group 1.
Because They Possess The Properties Of Metals, The Transition Elements Are Also Known As The
In chemistry, the term transition metal (or transition element) has three possible definitions: It has further been suggested by mott (1935, 1936a, 1936b) that the low conductivity of the transition elements, which are even worse conductors than the divalent elements, is due to another cause, namely, to the abnormal smallness of the free path. Most metals conduct electricity to a certain extent.
All Answers (4) The Conductivity Of Metal In Solution Depends On How Much The Metal Is Soluble In Solution.
The more solubility the metal is the more it conduct electricity, due to the presence of. Wills physical laboratory, university of bristol (transition metals</strong> ni, pd, and pt and of their alloys with cu, ag, and au have been discussed According to lenntech, pure manganese is reactive, burns in oxygen, rusts, and dissolves with dilute acids.
Their Electronic Structure Lets Them Form Compounds At Various Valences.
The metal’s atoms usually consist of valence electrons that are present in the atom’s outer shell and can also freely move about. Do transition metals conduct electricity. Metals conduct electricity because of the electrically charged electrons or particles.
Comments
Post a Comment